You may have seen a recent article making the rounds about chocolate bars containing high levels lead and other heavy metals. It made me wonder just how bad these are for your body, as I’ve eaten my fair share of chocolate this year. For instance, how much chocolate would you have to eat to have lead levels comparable to someone who grew up in the 70s? Did they even have that much lead in their blood compared to someone from ancient Rome? The chocolate bar with the most lead in it was Hershey’s Special Dark Mildly Sweet Chocolate, which contained 1.325 micrograms of lead per ounce, or about 5.6 micrograms per 4.25 oz. bar. Is this a dangerous level of lead to ingest?
It’s well known that the advent of leaded gasoline caused IQ loss in large cohorts of the American population, leading to an average loss of 2.6 points per person, (824 million IQ points are estimated to have been lost in total due to lead exposure) most concentrated in those born around 1960-70, some of whom saw damage as high as 7 points per person.
Recalling the concept of Route of Exposure from my hazardous waste engineering class, I decided to start looking at the differences between exposure via airborne particulate (as in car exhaust), ingested lead (as in chocolate) and exposure via dissolved lead (as would be most typical in the case of lead plumbing), as I thought this would help us estimate the impact of lead on the body based on environmental levels.
The lead absorption rate through the stomach ranges from 20-100% absorption, from full stomached adults to empty stomached children. It seems commonly accepted that the rate of absorption over large groups can be estimated around 70 percent. However, this rate is much higher for lead that is not already dissolved in water. Dissolved lead is absorbed at a 40-50% rate in children and only a 3-10% rate in adults.
In the case of inhalation, small particles are almost always completely absorbed, and large particles that are only partially absorbed are most often moved to the gastrointestinal tract by means of mucosal transport, where they undergo ingestion rates.
The average 3 year old child has about 1L of blood in their body, so to compare to the median child of 1970, they would need to absorb 150 micrograms of lead into their bloodstream. Assuming you fed them 27 Hershey’s Special Dark Mildly Sweet chocolate bars on an empty stomach, (7 pounds of chocolate, nearly a 3rd of their bodyweight!), then you would begin to see effects similar to those experienced by the median child in 1970s America. However, this lead would be mostly cleared from the blood within 2 months time, so it’s unclear how much damage it would do as compared to the constant exposure from 1970s air. My intuition is also that absorption rates would not come close to 100% due to the body already being semi-saturated after the first few bars were ingested, and most of the lead would pass through to excretion. So, the concern over lead in chocolate today has probably been overblown, but this lead me to wonder, were the Romans (who famously used lead in their plumbing systems) exposed to similar levels of lead to people born in 1970? What effects could this have had on Roman society and the minds of everyday people?
While the Romans did experience airborne lead pollution, it was not comparable to the scale of airborne pollution due to car exhaust in the 1970s, and came mostly from lead mining and smelting, which was concentrated heavily in Spain, Britain, Greece, and Asia-Minor. Out of 209 known roman mines that extracted lead, only 7 were in Italy. So I think we can rule out airborne lead pollution from our model of the Roman body.
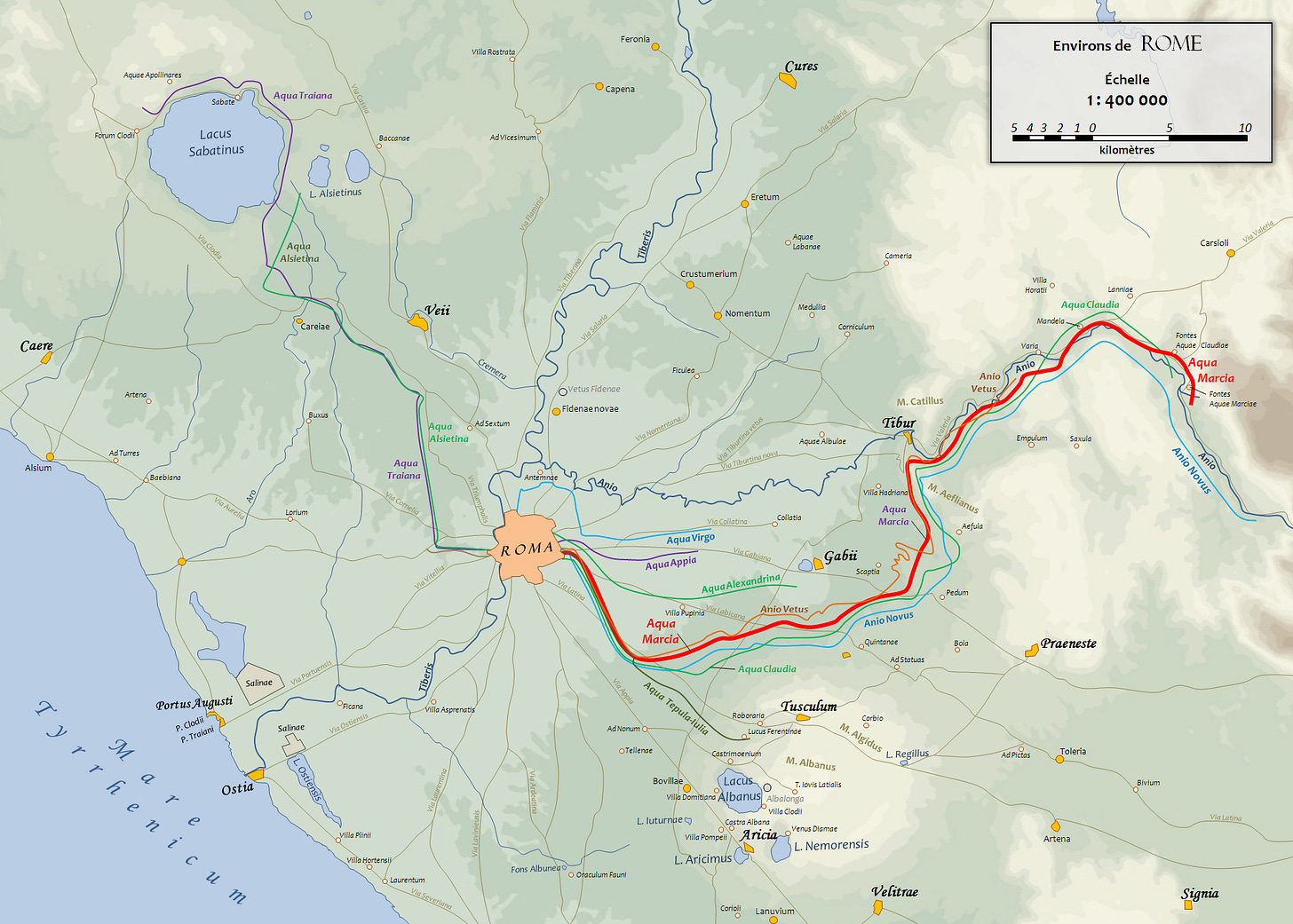
Next up would be the plumbing. Famously, Roman plumbing was done with lead pipes, but would this have been enough to poison the Romans? Probably not, and for a few reasons. While Roman aqueducts were sometimes lined with lead plates, and lead offshoot pipes were common in the empire, the water was never in contact with these surfaces for all that long. This is because the aqueducts did not function the same way that our modern plumbing systems do. In an aqueduct, water doesn’t sit still, and is instead always moving. Any water not used simply empties out of the lowest end, usually into a river, (In Rome’s case, the Tiber).
The longest of Rome’s eleven aqueducts, the Aqua Marcia, ran a length of 91 kilometers. With a cross section of only .81m^2, and a flow rate of 187,000 m^3 per day, we know that water spent less than 10 hours in contact with the lead plates (even less so due to some areas not being lined, tunnels, and flow not in contact with the plates), even in the longest of Rome’s aqueducts.
If we approximate the cross section to be square and assume that all the water was exposed to these plates and that the plates lined all sides of the aqueduct, then we’re looking at a contact area of 3.6m^2/m of length, or 327600m^2 of total contact area for the total length of the aqueduct.
I was about to go ahead and calculate the rate at which lead would have dissolved into Roman water using something called the Langlier Index and some rather complicated water chemistry but I came across something I think is rather important. The water in aqueducts was almost never in direct contact with the lead plating that lined them. This is because the mineral rich nature of the spring water that fed Rome’s water system. Over time, these minerals formed a deposit called travertine along the full surface of the aqueduct, meaning that after a few years of constant deposition, almost no lead would have been able to leech into the water. The travertine was occasionally scraped to improve flow rates, but this maintenance was irregular enough that on the whole I think it’s negligible.
Lead in Roman food and drink is where things gets interesting. Roman cookware, serving dishes, and cups were often made of or lined with lead, so I think we can expect rather high levels of the mineral in any processed food. Most interesting however is the use of lead as a sweetener in both Roman food and Roman wine. This was mainly in the form of lead acetate (flakes of which were sprinkled upon food) and the syrups Defrutum and Sapa.
Defrutum was a kind of sweet and tangy preservative, made by reducing a pulpy grape juice called must. This must was traditionally boiled in a lead-lined pot until half of it’s original volume remained.
Sapa was basically the fancier version of Defrutum, and was reduced to a third of it’s volume in much the same way.
There’s some disagreement about how common lead cookery was in Rome, but it seems commonly accepted that even when using copper cookware Romans often lined it with a soft lead alloy known as stagnum, and in recipes for Sapa and defrutum, a lead pot is almost always called for.
Modern attempts to recreate this Sapa resulted in a liquid with a lead content of 2900 micrograms per liter. (Although the proportions called for by some recipes (Columella) would place the concentration at nearly ten times that (21,000 micrograms/L)) Recall that as little as 150 micrograms absorbed by our three year old child was enough to cause large IQ effects, and a full grown man would need to absorb about 5 times as much to experience the same ratio in his bloodstream (750 micrograms) assuming the same rates of absorbency.
From Penelope’s blog at UChicago (which was an excellent resource in this endeavor)
Such levels of lead have significant physiological consequences. A single teaspoon of Columellan wine would have approximately 103 µg/dL of lead. As reported by the U.S. Department of Health and Human Services, there is brain and kidney damage in adults with blood lead levels of 100 µg/dL; gastrointestinal symptoms such as colic with levels of approximately 60 µg/dL; anemia with levels of 50 to 80 µg/dL; neurological symptoms with levels of 40 to 60 µg/dL; depressed sperm count with levels of 40 to 50 µg/dL; and increased risk of preterm delivery, low birth weight, and impaired mental development with maternal blood lead levels of 10 to 15 µg/dL. The physiological insult to children is even greater. Since 2012, the Centers for Disease Control and Prevention has recommended intervention in children five years or younger when the level of lead in the blood is above 5 µg/dL and even may lower that figure to 3.5 µg/dL.
Nriagu estimates the aristocracy of Rome to have consumed two liters of wine a day or almost three bottles (which would seem to make alcoholism more suspect than lead poisoning) and the resulting lead intake to have averaged 180 µg daily. He further estimates the total amount of lead absorbed from all sources to be 250 µg per day and lead concentration in the blood to be 50 µg/dL, at least for the gluttonous and bibulous (as he phrases it) and those with an appetite for adulterated wines and sweetened dainties—who he presumes most Roman emperors to have been.
Given that the Romans were famous for sweetening their wine with this Sapa, and drinking large quantities of wine, (the aristocracy estimated to be drinking 2 liters of wine a day!), we can assume their blood lead levels to be extremely high, even given the fact that many diluted their wine with around 3 parts water to 1 part concentrate.
Doing some napkin math:
2900mcg/L / 4 (to account for the dilution)
=725mcg/L in diluted wine
*2 liters a day among the aristocracy
=1500mcg of lead ingested per day
taking the 3-10% absorbency rate among adults we referenced earlier, we can peg the absorbed amount at between 45 and 150 mcg a day from wine alone.
(it should be noted that if drinking Columellan wine, you can almost 10x this number)
This is a good check on Nriagu’s estimate that Penelope’s blog references, so we’ll take those stats to be roughly accurate going forward.
Thus, with Nriagu’s estimated a 500mcg/L blood concentration, we know that the average roman man (with 5 liters of blood) had about 2500 mcg of lead in his blood, or ~3.5x more than the equivalent of 1970s level.
I am by no means a doctor, but I do know that each organ system has it’s on threshold of concentration for failure, so I would expect the effects of lead poisoning to compound rather than scale linearly, especially considering that as one organ system fails, others often fail in response. So 3.5x the most dangerous level we’ve experienced in living memory should be held as quite dangerous in my opinion.
So, now that we know the Romans were essentially poisoning themselves, what does that tell us? One interesting aspect of lead poisoning in antiquity is it’s class distribution. Because lead acetate sweetener cost money and concentrated wine was also more expensive than the dilute wine designated to the lower classes or the relatively lead free (but pathogen bound) water that the peasantry was afforded, it was the aristocracy, rather than the poor who experienced the effects of lead poisoning most acutely.
This is interesting because it’s essentially the inverse of the class-dose relationship we see today with environmental contaminants like lead paint and heavy cleaners. This unique concentration of poisoning among the ruling class lends itself to the theory that this poisoning may have contributed with the instability and eventual downfall of the Roman Empire, and has been much theorized about by historians, and although I agree that it’s effect on the Empire is largely overrated, I am still of the school of thought that institutional and environmental factors were more responsible for the empire’s decline.
I hope you enjoyed and learned something from this post! Don’t be afraid to eat chocolate from your Valentine this month they’re (probably) not trying to kill you, but if they break out the lead lined pot, run for the hills!
Until next time,
-Connor, OfAllTrades
And please share with your friends, family, and any other prospective readers you might know!
And if any of you readers out there are secretly crypto millionaires and would like to leave a tip, please check out https://alltrades.eth.xyz/