Fungus Alert: World Health Organization releases fungal priority pathogens list
Alarm bells sound on emerging fungal threats
On Tuesday, the World Health Organization rallied scientists across the globe in a call to increase R&D on a set of fungi they have classified as global threats to human health. Listed below are the 19 fungal threats they named as global research priorities, mostly due to their increasing prevalence, and their growing capabilities to resist the sparse selection of available antifungal treatments. These trends, along with the ongoing trend of thermoresistant emergence among commensal fungi, cast a looming shadow on the state of antifungal medicine. A new class of antifungal drug or a set of targeted vaccines may be needed to slow the rise of these emerging fungal threats.
If you’re an avid reader of this blog, you’ll recognize Candida auris and Cryptococcus gattii as old friends from past posts, which you can read for background here, and here.
In this post, I’ll summarize the WHOs findings, and explain how each of these fungal threats earned their place on the list, starting with Cryptococcus neoformans.
Cryptococcus neoformans
C. neoformans is endemic to the natural environment, usually living in soil or in decaying wood. In human infection models, the spores are inhaled, and initially infect the lungs. From there, it can spread to the central nervous system as well as the blood. The infection, cryptococcosis, mostly occurs in the immunocompromised, typically, HIV positive individuals or those taking immunosuppressants for organ transplant purposes. It has a mortality rate ranging between 41%-61%, and the infection can last 18-39 days. Complicating symptoms include increased intercranial pressure as well as renal failure. There is no vaccine for C. neoformans, so preventative measures are centered around reducing exposure and dose size, often by masking and avoiding dusty environments or where large amounts of wood is in decay. Treatments are sparse for C. neoformans, but include fluconazole, or in severe cases, a cocktail of amphotericin B ( a relatively broad-spectrum antifungal) and flucytosine, with fluconazole to follow. C. neoformans appears to already be growing resistant to fluconazole however, with reports of reduced effectiveness. Human-Human infection has not yet been observed.
Candida Auris
Candida auris is next on the list, and it’s climate change driven emergence was the main subject of one of my previous posts, which you can read here. Candida auris exists around the world, and is resistant to most antifungals, with some strains resistant across the lot of medicines available. Even more worrying, Candida auris is growing more and more thermoresistant each year. This is important because our immune systems are poor at differentiating between fungal and human cells, and so fever is one of the body’s favourite tools when dealing with fungal infections (this is partly why reptiles bask in the sun, as they cannot produce fevers of their own). With thermoresistant fungi, fevers are relatively ineffective. Once again, the immunocompromised are most at risk, and the pathogen can cause invasive candidiasis of the blood, heart, CNS, eyes, bones, and other internal organs. Mechanical ventilation is also a large risk factor, and C. auris is usually nosocomial (emerges in hospitals) and has high outbreak potential. Depending on what part of your body the fungus attacks, hospital stays can range 46-68 days, (and are about 2 times as long for children). There has been steady increase in C. auris outbreaks, even persisting throughout the COVID-19 pandemic. There is no vaccine for C. auris, and it’s difficult to detect or disinfect for. As stated above resistance to more common antifungals is high, due to C. Auris’ inherent levels of resistance. It is resistant at rates of 87-100% against fluconazole, and most vulnerable to treatment from echinocandins, which target the fungal cell wall. However, pan resistant strains have been described.
Aspergillus fumigatus
A. fumigatus is an extremely common environmental mold, which is inhaled, causing aspergillosis. The condition primarily affects the lungs, but can spread to the brain, CNS, and other sites as well. A. fumigatus has recently begun to develop resistance to Azoles. Azoles are a family of antifungal drugs including fluconazole and itraconazole, and attack fungi by interfering with the enzymes that create and repair their cell membranes, eventually disrupting them to the point that the fungal cells collapse. Mortality rates of those infected with azole resistant a. fumigatus usually range between 47-88%, but some studies have recorded 100% mortality, and the number of cases of azole resistance have been climbing. While no vaccine exists, preventability is still high. Antifungal prophylaxis for high risk groups has been shown to prevent invasive aspergillus and azole resistance is screen-able, allowing early detection in the immunocompromised. It’s thought that resistance to azoles may be due in part to their widespread use in agricultural fungicides, leading to resistance being selected for in environmentally latent strains. It is also thermoresistant, capable of growth at 37 °C or 99 °F (normal human body temperature), and can grow at temperatures up to 50 °C or 122 °F, with conidia surviving at 70 °C or 158 °F—conditions it regularly encounters in self-heating compost heaps. It is estimated that A. fumigatus may be responsible for over 600,000 deaths annually.
Candida albicans
C. albicans lives natively in the human microbiome on our skin, and in our mucosal layers. Once in their lives around 75% of women will suffer from vulvovaginal candidiasis (VVC) and about 90% of these infections are caused by C. albicans. The link between C. albicans and Crohn's disease has been investigated in a large cohort. This study demonstrated that members of families with multiple cases of Crohn's disease were more likely to be colonized by C. albicans than members of control families. In healthy conditions, it is not harmful. However, when the balance of this delicate environment is disrupted, it can multiply out of control and infect other organs and tissues. C. albicans is often triggered by antibiotic use, immunosuppression, hormonal imbalances, pregnancy, and diabetes. When it becomes invasive, it can attack the blood, heart, CNS, eyes, bones, and other organs. Once the invasion occurs, mortality can range from 20-50%, even with antifungals. There is no vaccine and as it exists latently on the body, exposure prevention is nearly impossible. Treatment usually involves echinocandins followed by azoles, and resistance is increasing but still uncommon.
Those are the top 4 fungal threats classified as the “Critical Priority Group”
To follow are the 7 listed in the “High priority group”
Candida glabrata (AKA Nakaseomyces glabrata)
C. glabrata is a commensal yeast (it lives on humans harmlessly in normal conditions) that similar to C. albicans becomes opportunistically hostile to the body. It has a mortality rate of 20-50% upon invasion, and it has been increasing not only in frequency relative to past C. glabrata infections but also as a proportion of all Candida infections. A high dose of azoles is needed to combat it, which can cause complications and side effects, especially in children. C. glabrata is slowly becoming resistant to echinocandin treatments as well. An experimental, but effective second-line treatment for chronic infections, is the use of boric acid.
Histoplasma spp.
Histoplasma is an environmental fungus, that likes to live in soil rich in bat or bird droppings. It’s found in the central and eastern U.S., mainly in the Ohio and Mississippi River Valleys, as well as parts of Central and South America, Africa, Asia, and Australia. It has a high potential to cause outbreaks, especially among the immunocompromised, but does not spread person to person. The spores are inhaled through the lungs and can also attack the CNS, blood, and more rarely other parts of the body. Among the immunocompromised, mortality is ~10%. In healthy children, the mortality rate as been reported as high as 2.7%. There is no vaccine available, however, healthy patients often recover without treatment. For severe cases, amphotericin B and itraconazole are recommended. As it stands, Histoplasma spp. is only moderately resistant to these treatments.
Eumyceotoma causitive agents (ECA)
Eumycetoma is a deep tissue infection caused by a wide range of fungal pathogens which act in similar ways, that enter the body through breaks in the skin. It often occurs in those with disabilities or diabetics, as well as the global farming poor who have high incidences of exposed skin breaks, especially on the feet and legs when working in fields. Risk factors include being a farmer, male, and young. (11-30 years). Tropical areas report the most cases, but as with many of these fungal threats, the data is not complete and surveillance is difficult. Once the colony is established in the deep tissue, amputation rates can be as high as 39%. The best thing you can do to prevent Eumycetoma is to wear shoes and engage in a daily hygiene routine. Because the condition is caused by so many different species of fungi, there is serious lack of data about their antifungal susceptibility. The heterogenity of cases suggests that some species of ECA are developing resistance but others are not.
Mucorales
Mucorales is another large group of fungi, consisting of different genera. They are globally distributed pathogenic molds, and cause a wide spectrum of infections, called mucormyosis, which affects skin and soft tissue injuries primarily among the diabetic and otherwise immunocompromised. Once it becomes invasive, Mucormycosis is a very serious disease, with mortality ranging between 20-80% in adults, and 72.7% in children. It’s been increasing in frequency over the last ten years, and is hard to prevent. There is no vaccine for fungi in the Mucorales group. They are inherently resistant to fluconazole, voriconazole, and echinocandins, and require large doses of amphotericin B to treat.
Fusarium spp.
Fusarium spp. are a group of globally distributed pathogenic molds, with high concentrations in the tropics. They are saprotrophs, living mostly in soil and decomposing organic matter and plants. When invasive to the human body, Fusarium spp. primarily targets the lungs and eyes, but have also been known to spread to the CNS. It can grow as yeast like structures in the blood, and pass undetected, delaying diagnosis until it grows into its hyphaetic form. Fusarium is the second or third most common invasive mold infection in immunocompromised patients. Once invasive, Fusariosis has a mortality rate between 43-67%, and are especially high for the species F. solani and F. proliferatum. Fusarium has been observed to be developing resistance to antifungals with the exception of amphotericin B.
Candida Tropicalis
Similar to the other commensal yeasts, Candida Tropicalis has pathogenic potential, and becomes life threatening upon invasion. Mortality rates upon invasion range as high as 55-60% in adults and 26-40% in children. Candida Tropicalis notably puts neonatal ICU patients at risk, as well as the other typical immunocompromised victims. It’s very hard to prevent, and some studies have reported C. tropicalis to have developed resistance to azoles at rates of up to 80%. For this reason, invasive disease is usually treated with echinocandins.
Candida Parapsilosis
C. parapsilosis presents a particular problem because of its propensity to form biofilms, which can cause infections around IV sites, which can be a point of entry for the fungus to enter the body. It has a mortality rate of 20-45% once invasion occurs, and similar to C. tropicalis, is a concern for neonatal ICUs. It’s thought that C. parapsilosis derives most of its antifungal resistance from it’s biofilm ability. Primary treatment is echinocandins.
Lastly is the medium priority group. (Do I detect a hint of priority inflation?) Some of these fungi are just as deadly as the previous ones if left untreated, but are either less globally abundant than others, or are at much earlier stages of developing resistance to antifungal drugs. You can see the list of aspects considered by the WHO report below:
Scedosporium spp.
Scedosporium spp. are a family of globally distributed pathogenic mold, which take a filamentous form, and predate mainly on those with malignancy, HSCT (Hematopoietic stem cell transplants), and severe infection. In the wild, they live mainly in soil, sewage, and polluted waters. It is often confused with other filamentous fungal infections, and is thought to be under rated for that reason. It is highly resistant to all available antifungals, and has no pharmacological breakpoints. Once invasive, it imposes a mortality rate as high as 46% in adults. The most active antifungal agent to use against it is Voriconazole, but as stated above, it is quite resistant. Preventability is low and there is no vaccine. Once it reaches the CNS, it’s mortality rate spikes to 90%, and in extreme cases it can cause abscesses in the brain. The infection starts in the lungs and is often contracted by those who experience near or partial drowning in polluted waters.
Lomentospora prolificans
Lomentospora prolificans is an opportunistic mold. In adults and immunocompromised children it has over a 50% mortality rate upon invasion. However, it also routinely infects immunologically normal patients and presents as subcutaneous lesions following abrasions from contaminated splinters of thorns. In normal cases, the infection remains localized, with some characteristic bone or joint involvement. However, in the immunocompromised, the infection often becomes invasive and disseminates throughout the body. Other than limiting exposure events, preventability is low, and there is no vaccine. Antifungal resistance is high, and all current licensed antifungal agents have no in vitro activity against this fungus. However, the new drug Olorofim is a beam of light in this otherwise dark picture.
Coccidiodes spp.
Coccidiodes spp. are some of the most virulent fungal pathogens. The spores are inhaled from the environment, and the infection is initially based in the lungs. Coccidiomycosis (AKA San Joaquin Valley Fever) is largely confined to the Western Hemisphere and is endemic to the South Western U.S. For most, (about 60%, infection is asymptomatic. The most common clinical syndrome in the other 40% of infected patients is an acute respiratory illness characterized by fever, cough, and pleuritic pain. Skin manifestations, such as erythema nodosum, are also common with Coccidioides infection. Coccidioides infection can cause a severe and difficult-to-treat meningitis in AIDS and other immunocompromised patients, and occasionally in immunocompetent hosts. Infection can sometimes cause acute respiratory distress syndrome and fatal multilobar pneumonia. The risk of symptomatic infection increases with age, and is especially prevalent among African Americans, and those who are exposed to lots of dust and soil particles. Upon invasivity, the mortality rate is 2-13%, and are much higher in more vulnerable patients. Preventability is low with the exception of reducing dust inhalation. There is no vaccine available. Testing for antifungal resistance has been slow because of the danger to lab staff, but it currently responds well to antifungals with the exception of fluconazole, which requires a high minimum dose to treat coccidioidomycosis.
Pichia kudriavzeveii (Candida krusei)
P. kudriavzeveii is another opportunistic yeast, that is found all around the globe and lives as part of the human microbiome, it is also used in chocolate production to ferment cacao beans. Once invasive, it has a mortality rate of 44 to 67% in adults. It is naturally resilient to fluconazole, but is still affected normally by other azoles and echinocandins. Interestingly, it is the only member of the medically relevant candida family that can grow without extra vitamins such as biotins. According to a 2015 paper, patients that obtain this fungus as a disseminated infection have the lowest 90 day survival rate (~53%) of all known candida species.
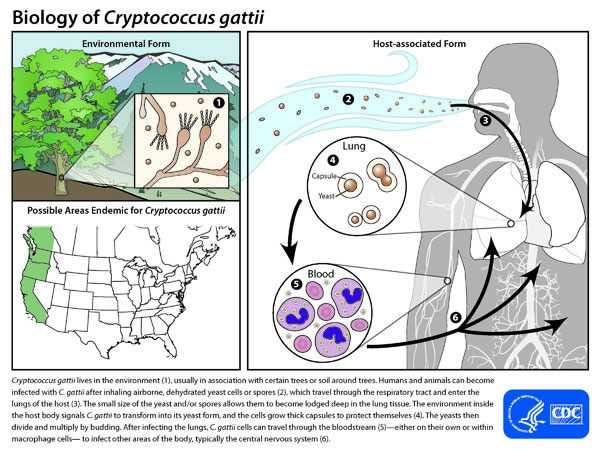
Cryptococcus gattii
Cryptococcus gattii is one of the two fungi I’ve written about before regarding their potential as threats to human health. While it is globally distributed among forests, it is more frequent in tropical and subtropical environments, especially where there is high humidity. Prevalent along the North American West Coast, it accounts for 10-33% of all cryptococcal infections. Risk factors include working in forestry or being an outdoorsy person, especially in the Pacific North West, and being immunocompromised. Luckily, rapid tests have recently been made available, allowing for early diagnosis. Early treatment is relatively effective, but once the infection becomes invasive, mortality can be between 10-25%. No vaccine is available. Because C. Gattii had evolved in contact with many soil amoebas, it was already adapted to fight human white blood cells extremely well, and can even reproduce within macrophages like white blood cells. Both are eukaryotic, and attack their prey via phagocytosis, wherein the amoeba or white blood cell engulfs the bacteria or fungi and attempts to digest it. Similar to the phenomenon we observed in Candida Auris, whereas the internal temperature (resting and during fever) of our body would normally protect us in tandem with an immune response, this has become less effective as the environmental C. Gattii strains have adapted to be able to survive spikes of high temperature in the environment. People infected with C. Gattii need at least 6 months of treatment, and once masses form in the lungs or the brain, they often require surgical removal, which can be very risky in the case of a person with an immune system weakened by infection.
Talaromyces marneffei
T. Marneffei is a pathogenic dimorphic fungus endemic to SE Asia and some areas of China. It lives in the soil and among decaying wood, and gets to the human body by means of spore inhalation. The disease is considered an AIDS-defining illness.Although both the immunocompetent and the immunocompromised can be infected, it is extremely rare to find systemic infections in HIV-negative patients.Talaromyces marneffei has been found in bamboo rat faeces, liver, lungs and spleen. It has been suggested that these animals serve as a reservoir for the fungus. It is not clear whether the rats are affected by T. marneffei or are merely asymptomatic carriers of the disease. One study of 550 AIDS patients showed that the incidence was higher during the rainy season, which is when the rats breed. But this season also has conditions that are more favorable for production of fungal spores (conidia), which can become airborne and be inhaled by susceptible individuals. Another study could not establish contact with bamboo rats as a risk factor, but exposure to the soil was the critical risk factor. However, soil samples failed to yield much of the fungus. It is not known whether people get the disease by eating infected rats, or by inhaling fungi from their feces. It is not yet resistant to any antifungals, but it does require high doses of Fluconazole, Anidulafungin, and caspofungin to treat, indicating that it may have some inherent resistance to those already.
Pneumocystis jirovecii
Pneumocystic jirovecii is an opportunistic fungal pathogen which spreads person to person through the air. Every tested primate, including humans, appears to have its own type of Pneumocystis that is (for now) incapable of cross-infecting other host species and has co-evolved with each species. It is globally distributed and importantly, can be carried by healthy and asymptomatic individuals. It causes p. jirocecii pnemonia (PJP) which has a substantial but highly variable mortality range (up to 100% in some studies of immunocompromised individuals). Immunocompromised patients such as those with HIV, organ transplant patients and others taking medications that weaken the immune system are more affected. Risk factors for PJP include AIDS, cancer, iatrogenic immunosuppression with solid organ transplantation (especially renal), autoimmune and inflammatory disease, and nephrotic syndrome. Preventative drugs have been quite effective at stopping PJP from spreading, and antifungal resistance is yet to be investigated. Treatment of PJP usually involves different drugs to other types of fungal infections, with the PJP specific arsenal including Trimethoprim/sulfamethoxazole, pentamidine, and dapsone.
Paracoccidiodes spp.
Last but not least is Paracoccidiodes spp.. This family of fungi are endemic to Central and South America and invade the body either by way of spore inhalation or through abrasions to the skin. It has been found in high concentrations in the soil from armadillo burrows and in soils where coffee is cultivated, but it has not been otherwise linked to any specific soil environment. The endemic areas are characterized by hot, humid summers, dry temperate winters, average annual temperatures between 17 and 23 °C, and annual rainfall between 500 and 800 mm. It mainly affects the lungs, mucous membranes, and skin, but can spread to the lymphatic and reticuloendothelial system. Most people infected never develop any symptoms and it cannot spread between people. Risk factors include being over 40, working outdoors, and male. The hormone estrogen is thought to inhibit the transformation of the mycelial to the yeast form, as supported by in vitro experimental data, and this factor may account for the relative resistance of women to infection.When the infection becomes invasive and results in the condition of Paracoccidioidomycosis, the mortality rate ranges between 3-23%, higher in patients with HIV. It can present with complications such as low sdrenal reserves and lymphedema. It is usually treated with itraconazole, amphotericin B, or cotrimoxazole.
So what’s next? How can we prevent these emerging threats from breaking through the lines? New antifungals will need to be developed. Luckily, there are some coming down the line, but these will only stave off the marauding mushrooms.
These new drugs include Scynexis Inc.’s ibrexafungerp and Pfizer Inc.’s fosmanogepix, which have been shown in clinical trials to be effective against Aspergillus and Candida infections. Also on the way is rezafungin from Cidara Therapeutics Inc. (stuck in FDA approval process) to treat Candida infections. Drugs targeting the Class II dihydroorotate dehydrogenase (DHODH) proteins of L. prolificans, Scedosporium, Aspergillus and other rare moulds are the basis for at least one new therapy, Olorofim, which is currently in phase 2b clinical trials and has received breakthrough status by FDA.
However, it’s likely that fungi will only continue to grow resistant to these new drugs, many of which are only riffs on old formulations and do not really represent a new class of treatment. I am hopeful that the advent of new rapid manufacture vaccine technology may help combat this issue.
Until next time,
-Connor, OfAllTrades
Don’t forget to subscribe, it’s free!
And please share with your friends, family, and any other prospective readers you might know!
If you’d like to download the report yourself: see the link below:
https://www.who.int/news/item/25-10-2022-who-releases-first-ever-list-of-health-threatening-fungi











Terryifyig but thanks for writing.